Scientists crack green hydrogen code with iridium-free electrolyzer design – Interesting Engineering.
When used in an anode, the catalyst was stable for over 600 hours at high energy density, a record for cathode-based catalyst.
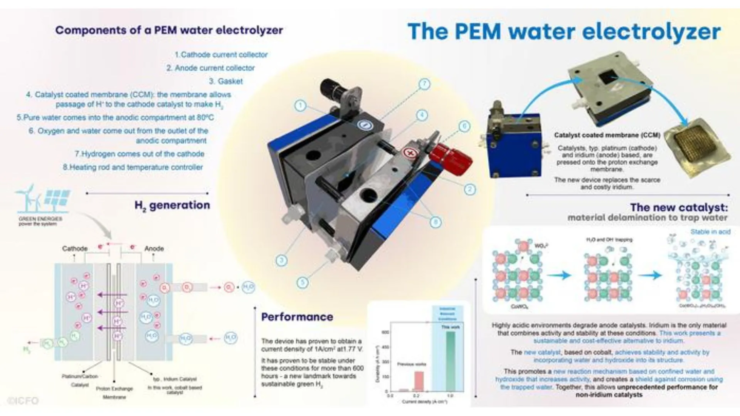
A collaboration between researchers at various institutes in Spain has successfully developed an electrolyzer that does not use the rare earth element iridium at its anode.
Under laboratory conditions, the team set records for the highest stability and energy density for an iridium-free electrolyzer, a press release said.
Hydrogen holds potential as a non-carbon emitting green fuel that could even fly airplanes someday. However, current production methods of hydrogen are fossil-fuel dependent and result in carbon emissions.
Therefore, researchers globally have been working on developing non-polluting ways of generating hydrogen. The proton-exchange membrane (PEM) approach of water electrolysis has reached high levels of efficiency. Using cleaner sources of electricity to power the device, one can also generate green hydrogen.
Electrolyzers, too, have drawbacks since the catalysts used in the reaction, such as iridium, are extremely rare on the planet. So, the researchers in Spain were keen to find a suitable alternative that could make iridium-free electrolyzers a reality.
Why is iridium so important?
Electrolysis is a complex reaction, and hydrogen production takes place in highly acidic environments. Under conditions of low pH and high electric potential, metals are not stable and tend to dissolve in the reaction, no longer acting as catalysts.
Iridium oxide, on the other hand, displays high stability in acidic environments while also retaining high activity levels, making it the metal of choice in PEM electrolyzers.
Previous research on iridium-free catalysts has led researchers to materials such as manganese and cobalt oxides. However, these materials did not work satisfactorily at scale, making them unsuitable for wide deployment.
A new approach
Researchers led by F. Pelayo García de Arquer, a professor at ICFO-The Institute of Photonic Sciences wanted to develop a catalyst based on cobalt, since it is abundantly available. However, due to previous failures in the approach, the researchers an innovatively different approach.
García de Arquer in a press release:
We designed a new material that actively involves the ingredients of the reaction (water and its fragments) in its structure.
The team used a process called delamination where part of the material was replaced by water, making the cobalt catalyst more robust than before.
García de Arquer added:
“We found that the incorporation of water and water fragments into the catalyst structure can be tailored to shield the catalyst at these challenging conditions, thus enabling stable operation at the high current densities that are relevant for industrial applications,”
The team worked with cobalt-tungsten oxide (CoWO4 or CWO) and used water to replace the tungsten oxide in the lattice structure. The catalyst was then used in the anode electrode during electrolysis and found to perform better and more stable than any prior catalysts made from cobalt.
This was because the removal of tungsten oxide left a hole behind, which was filled by the water and hydroxide ions. This prevented the cobalt from dissolving in the reaction and protected its stability. The catalyst worked for more than 600 hours, breaking previous records achieved with the material.
This is far from the stability of commercially available PEMs, but the work has given the researchers hope that they can work with more suitable candidates in the future.
Garcia de Arquer said in the press release:
“We will go through the whole the periodic table, if necessary. And we are going to explore and try with them this new strategy to design catalysts that we have reported in our study,”
READ the latest news shaping the hydrogen market at Hydrogen Central
Scientists crack green hydrogen code with iridium-free electrolyzer design – Interesting Engineering. source